News
A variety of endoscope products fill the gap in the industry and have developed for nearly 40 years. How does this company achieve domestic substitution?
- Categories:Company news
- Time of issue:2021-12-21
- Views:
(Summary description)According to the China Statistical Yearbook, in 1999, the average annual wage in society was 8,346 yuan. In the same year, the Liaoning Yearbook recorded that Shenda's endoscope sales revenue reached 15 million yuan. At the time, it had only 170 employees.
A variety of endoscope products fill the gap in the industry and have developed for nearly 40 years. How does this company achieve domestic substitution?
(Summary description)According to the China Statistical Yearbook, in 1999, the average annual wage in society was 8,346 yuan. In the same year, the Liaoning Yearbook recorded that Shenda's endoscope sales revenue reached 15 million yuan. At the time, it had only 170 employees.
- Categories:Company news
- Author:
- Origin:
- Time of issue:2021-12-21
- Views:

According to the China Statistical Yearbook, in 1999, the average annual wage in society was 8,346 yuan. In the same year, the Liaoning Yearbook recorded that Shenda's endoscope sales revenue reached 15 million yuan. At the time, it had only 170 employees.
Roughly estimated, the income generated by each employee in the year is about 88,235 yuan. This value is almost 10 times the social average annual wage level.
What does the Shenda endoscope, which created this brilliant achievement 20 years ago, look like 20 years later?
The endoscope track is hot, and financing of over 100 million yuan emerges one after another
With the aging of the population, the continuous improvement of health awareness, and the continuous upgrading and iteration of technology, the growth of medical demand is further promoted. According to Frost & Sullivan, the global medical endoscope market is expected to reach USD 39.6 billion in 2030. The size of China's domestic market is expected to grow from 23.1 billion yuan in 2020 to 62.4 billion yuan in 2030.
The continuous improvement of the market scale, coupled with the further emphasis on nosocomial infection by the Department of Infection under the epidemic, has ushered in a new wave of entrepreneurship in the field of endoscopy.
According to the statistics of Tianyancha, from January 2020 to the end of November 2021, there were 1,269 pan-related newly established enterprises in the field of endoscopy.
Capital is also pouring into this popular track, and large-scale financing of over 100 million yuan frequently appears in the field of endoscopy.
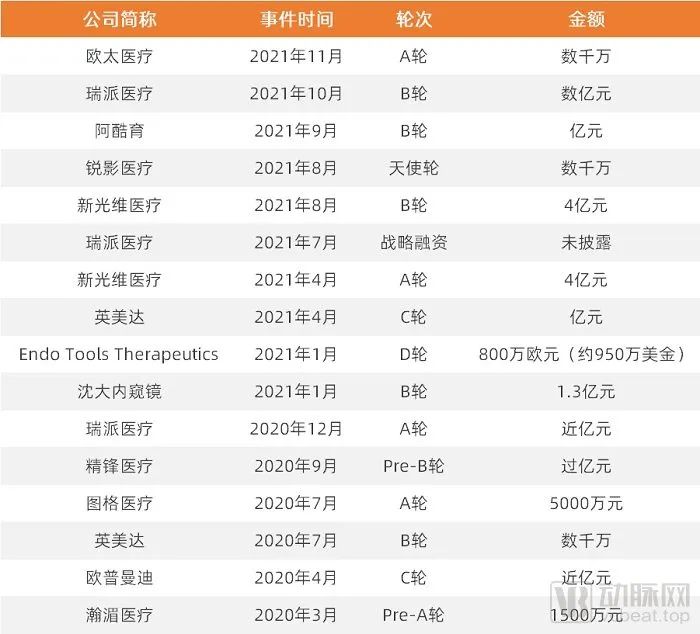
Arterial network is organized and mapped according to public information
The capital market is undoubtedly looking forward to the next IPO in the field of endoscopy.
Facts have not lived up to expectations. This year, the field of endoscopy has ushered in the grand event of Xinguang Medical's Hong Kong stock offering prospectus and the listing of Aohua Endoscopy Science and Technology Innovation Board. But it is still unclear who will be the next endoscopy company to submit a prospectus to an IPO.
While capital is continuously investing and watching the endoscope market, we have also seen an endoscopy company that is very familiar to the outside world and can be called an "old brand" from the companies that have obtained financing - Shenda Endoscopy.
In January this year, Shenda Endoscopy completed the B round of financing of up to 130 million yuan, which was jointly led by Jinding Capital and Chuangrui Investment, and joined by Hangshi Investment.
Shenda Endoscopy once stated that this round of financing "will be mainly used for the research and development and upgrading of new products and channel construction, as well as continue to build a leading brand of domestic hard mirrors".
For Shen Da Endoscopy, the IPO has been put on the agenda.
As an enterprise established in the 1980s, Shenda Endoscopy has repeatedly filled the industry gap in China.
The investor of this round of financing of Shenda Endoscope once said, "Shenda Endoscope has rich technical accumulation and innovative R&D and upgrading capabilities in the field of domestic hard mirrors. Shenda Endoscope relies on its characteristic product solutions and forward-looking The upgrading of the sex industry is expected to become a leader in technological innovation and minimally invasive applications in the field of domestic replacement of endoscopes."
What kind of "technological precipitation and innovative research and development" does this company, which has been developing for nearly 40 years, make capital choose to invest and follow up?
How did Shenda endoscopy go from a blank period in the industry to today?
With doubts and curiosity, we "approached" Shenda's endoscope, trying to lift the veil over the head of this company that rarely speaks to the outside world.
Arterial Network interviewed Jiang Shouwang, Chairman of Shenda Endoscopy, and CTO Chen Chong, and listened to their stories about the past and future of Shenda Endoscopy.
The first domestic endoscope was born, laying the foundation for the development of Shenda endoscope
When talking about the history of development, Jiang Shouwang, chairman of Shenda Endoscopy, mentioned another important figure who laid the foundation - he is Shenda Endoscopy who has won the title of "Scientist with Outstanding Contributions" and enjoys special allowances from the State Council. Founder Jiang Kerang.
Jiang Kerang, born in 1935, graduated from the Philosophy Department of Liaoning University in 1959.
How can a philosophy student understand the repair and manufacture of these precision instruments?
In fact, Jiang Kerang spent a long "study period". Almost "self-taught", he started repairing optical instruments such as geodetic instruments, theodolites, levels, and microscopes, and gradually shifted from repair to assembly and design. Soon, his watch and instrument company was changed to Shenyang Dadong Optical Instrument Factory, and he became the main technical person in charge.
It may be a coincidence that he came into contact with an endoscope, but it may not be just a coincidence that Jiang Kerang finally chose this path.
In 1975, Jiang Kerang met Wang Tingrui, director of the gynecology department of the 202 Hospital of the Military Region. At that time, Director Wang was studying hysteroscopy. Director Wang only has a photo published in the American Journal of Obstetrics and Gynecology - an unshaped sample developed by a family planning research center in the United States. Jiang Kerang followed this picture model and tried again and again, starting from solving the problems of material, structure, lens processing and design, etc., and finally ushered in the dawn of victory after experiencing repeated failures.
In 1976, the X-G3 hysteroscope was successfully developed and passed the appraisal. This move fills the gap in the domestic endoscopy field.
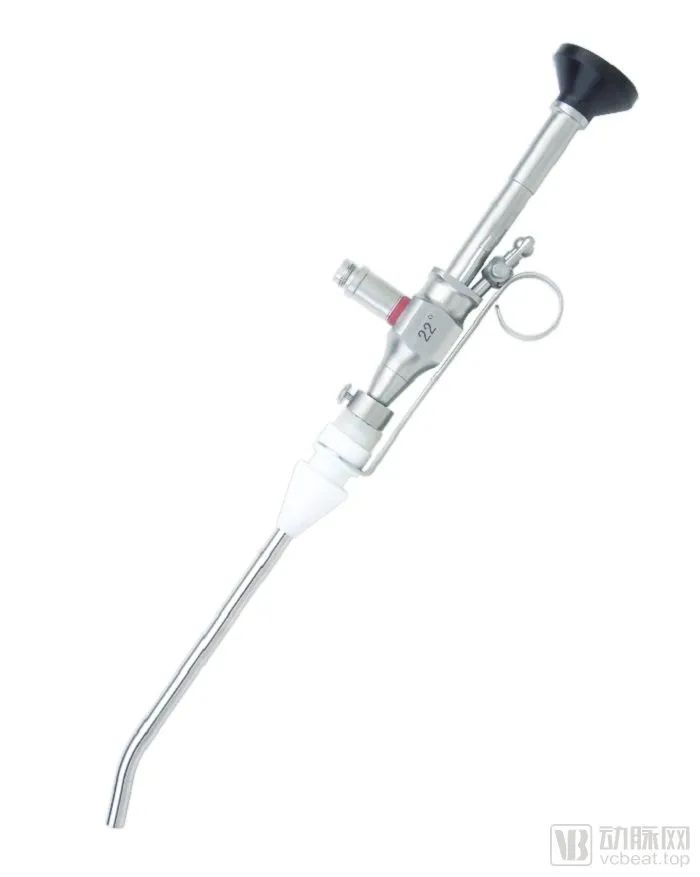
Today's "XG-5/XG-5A Hysteroscope" of Shenda Endoscope
Various awards are also coming. In 1978, he won the National Science Congress Award.
But he didn't stop, and then—
Arthroscopy, the third prize of major scientific and technological achievements in Liaoning Province, and the second prize of scientific and technological achievements of the Chinese People's Liberation Army;
YJ-1 type amniotic fluid mirror, the third prize of Shenyang scientific and technological achievements;
...
For Jiang Shouwang, chairman of Shenda Endoscopy, the inventions and innovations made by founder Jiang Kerang have laid the foundation for the development of Shenda Endoscopy. Looking back today, nearly 40 years later, the development of Shenda endoscopy can be roughly divided into the following stages:
Phase 1: 1985-1990. This stage is the period when Shenda endoscope established its own industry status. As an inventor, Jiang Kerang helped Shenda endoscope to establish its own industry position through the products developed by himself, including curved tube type hysteroscope.
The second stage: 1991-2000. Shenda Endoscopy started the road of multi-department product expansion, and gradually expanded from gynecology to urology, and took the lead in launching products such as "ureteroscopy" in China. Its related urological endoscopy products won the support of the National Urological Association at that time, and the products started to be sold quickly. Shenda Endoscopy has also built cystoscopes, resectoscopes, ureteroscopes and other ancillary products around the urology department.
The third stage: 2001-2018. Shenda Endoscopy has gradually established its market position. Since 2001, Shenda Endoscope has gradually covered products from more than 10 departments including gynecology, urology, ENT, general surgery, anorectal, neurosurgery, etc. Covered in many hospitals. As of 2018, Shenda endoscopy has obtained more than 60 registered patents in related fields. During this period, Shanghai Jiawang Endoscope Technology Co., Ltd. was established in Shanghai, which laid the foundation for the electronic endoscope of Shenda Company.
The fourth stage: 2018 - present. While precipitating endoscope development technology, Shenda Endoscope strives to benchmark the leading products of international leading companies, and realize domestic substitution of imported products through industrial upgrading.
As a veteran endoscopy company, Shenda Endoscopy has had many glorious moments. In addition to designing the first endoscope in China for the first time to fill the gap in the industry, Shenda Endoscope is also committed to creating better products and finally realizing domestic substitution. In the process of starting from scratch and from being to being refined, Shenda Endoscopy has gradually built its own competitive advantages and its own technical barriers.
Multi-pipeline products go hand in hand, and a number of patents build R&D barriers
Shenda Endoscope currently produces more than 2,000 varieties and specifications of various rigid endoscopes and supporting products, which can meet the requirements of urology, gynecology, general surgery, orthopedics, ENT, anorectal, neurosurgery, minimally invasive surgery demand.
Jiang Shouwang pointed out, "The sole goal of Shenda Endoscope is to become a world-class endoscope manufacturer."
Being able to let him say such words, allowing Shenda endoscopes to operate in multiple departments, and to create products that cover almost all departments, is closely related to the advantages that Shenda endoscopes have gradually built up.
Jiang Shouwang said, "The manufacture of endoscopes involves the comprehensive application of multiple disciplines. Shenda endoscopes have been involved in this field for nearly 40 years and have accumulated a lot of advantageous experience."
First of all, Shenda's endoscopes are deployed in the field of rigid endoscopes and have accumulated a large number of endoscope intellectual property rights. "From product development to industrial production, we have summed up a lot of product development and production experience and formed a relatively complete independent intellectual property system." At present, Shenda Endoscope has more than 60 product registration certificates and more than 60 patents. The patents cover the European Union, the United States, Japan and other countries and regions. It not only builds competition barriers for Shenda endoscopy, but also provides a "pass" for Shenda endoscopy to develop overseas markets.
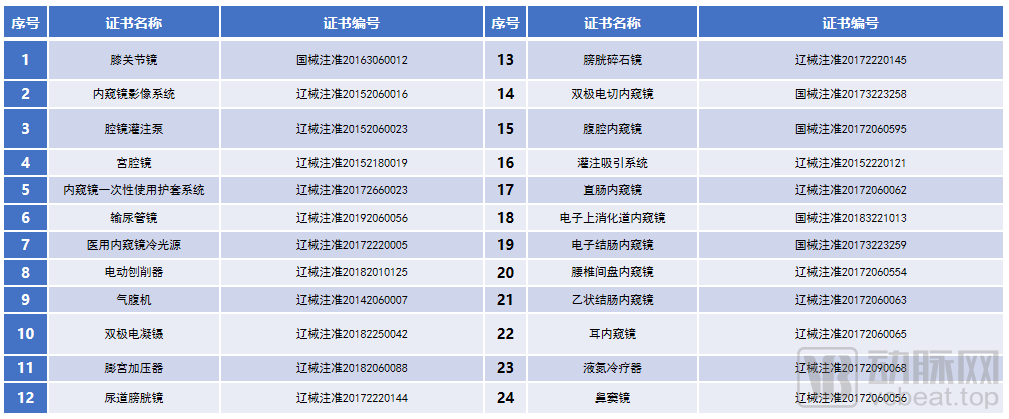
Shenda endoscope part of the medical device registration certificate
Chen Chong, CTO of Shenda Endoscopy, added, "Shenda Endoscopy is one of the few companies that can cover the entire industrial chain. From product design to product process, structural design, and then to mass production, Shenda Endoscopy All have independent intellectual property rights. This has created technical barriers and product barriers for Shenda endoscopes."
Secondly, the clinical effect of Shenda's endoscopy products has been verified. Shenda endoscopy has covered a large number of hospitals of all levels in the past 40 years. "In the 1980s and 1990s, when we promoted endoscopes, most doctors did not use them. Now basically every department uses endoscopes." Jiang Shouwang also pointed out that compared with the expensive endoscopes imported from abroad The development of domestic endoscopy equipment has fully guaranteed the use of some hospitals with insufficient funds. At present, the clinical feedback of doctors in hospitals at all levels who use Shenda endoscopes is good. With the improvement of technology and quality, he also believes that the coverage of domestic endoscopy equipment in more tertiary hospitals will be further improved.
At present, Shenda Endoscope is also actively deploying many innovative fields, including ultra-high-definition endoscopes (Shenda Endoscope has mastered the unique domestic laser welding, sealing, micro lens gluing and other core technologies. The gap between the scope and the international first-class brands lies in the processing technology of aspherical microlenses), neurosurgical endoscopes, microprobe digestive ultrasound endoscopes, confocal microendoscopes, high-definition 4K endoscope camera systems, fluorescence camera systems and other products .
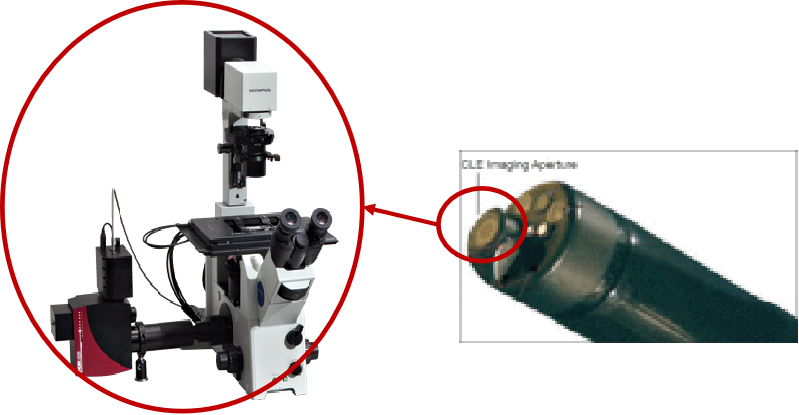
In confocal endoscopy products, the "microscope" is concentrated at the tip of the endoscope
Taking confocal endoscopy products as an example, it is composed of a confocal laser microscope and a traditional electron endoscope. On the premise of applying a contrast agent, it acquires confocal images through a low-power laser. This technology can achieve 1000 times magnification of tissue, and can be widely used in the early diagnosis of tumors in natural orifices such as the digestive system and respiratory system. It can obtain histological images of the surface and subsurface (mucosa) structures in the body without sampling and biopsy. , for real-time high-resolution histopathology studies. While helping to improve the accuracy of biopsy, this product can reduce the missed detection rate and has considerable development potential.
For CTO Chen Chong, the advantages of Shenda endoscope in the product pipeline are obvious.
First, in terms of traditional endoscope operation and manufacturing, Shenda's endoscope has maintained a relatively high level of market share and high praise from clinicians. "When many hospitals/doctors buy endoscopy products, Shenda endoscopy products are often the first choice of domestic brands."
Second, Shenda endoscope has carried out innovative layout in many products such as image host and confocal endoscope. Shenda Endoscope has been carrying out innovative research and development and investment in the field of endoscopy for many years. Some of these products have already completed research and development and have entered the stage of clinical registration testing.
Behind the multi-pipeline product layout, Shenda endoscope pays more attention to the follow-up of clinical needs.
Starting from clinical needs, cooperate with many scientific research institutes
Perhaps from the invention of the first endoscope product by Jiang Kerang as the founder, we can see the shadow of the combination of medicine and engineering in the early development of Shenda endoscope.
Shenda Endoscopy, which has been developing for many years in the field of endoscopy, always adheres to the clinical needs and develops endoscopy products that meet the clinical needs of doctors through the combination of medicine and industry.
In order to better create products that meet clinical needs, Shenda endoscopes choose to cooperate with well-known hospitals/doctors experts or scientific research institutes in the research and development to achieve the deep integration of industry, university and research.
In terms of hospital doctors, for example, Shenda Endoscopy and Academician Gu Ying of Beijing 301 Hospital, Wang Ningli, Dean of the Academy of Ophthalmology of Capital Eye University, He Zhisong, Chief Physician of Urology Department of Peking University First Hospital, Wu Anhua, Director of Neurosurgery of China Medical University, Xi'an Jiaotong University Experts and professors such as He Dalin, director of the Department of Urology of the Affiliated Hospital of the Medical College, and other experts and professors have reached the endoscope research and development projects related to each department.
In terms of scientific research institutes, for example, Shenda Endoscopy and the Suzhou Institute of Biomedical Engineering Technology of the Chinese Academy of Sciences have jointly developed "Confocal Microendoscopy" and "Microprobe Digestive Ultrasound Endoscopy Imaging System Project"; The University of Science and Technology jointly developed the "Multifunctional High-Frequency Electrosurgery Project"; cooperated with the Shenyang Institute of Automation, Chinese Academy of Sciences and Beihang University to develop the "R&D and Clinical Application of Single-port Endoscopic Surgical Robot System" project.
Overseas, Shenda Endoscopy has also cooperated with a German research institute to develop "the initial optical system design of laparoscope and the development of high-precision micro-aspheric lens technology", "the initial optical system design of arthroscope and high-precision micro-aspheric lens technology" Technology Development" project.
CTO Chen Chong introduced that in order to better build high-end endoscopy equipment, Shenda Endoscopy has been strengthening its own team building and increasing investment in research and development. In order to better cultivate talents, they chose to build their own talent echelon through the "old and new" approach. At the same time, due to the need for compound talents in the field of endoscopy, Shenda Endoscopy is also actively introducing talents in various fields such as optics, machinery, electronics, and digitalization.

Shenda endoscope production workshop
Chen Chong mentioned that there are two modes of cooperation with scientific research institutes: one is entrusted research and development, and the other is joint research and development.
"We focus on independent research and development, and the direction of research and development is often combined with clinical needs or cutting-edge trends in clinical academics. Focusing on clinical needs, we upgrade the performance of existing products, and deploy and expand future products." He particularly It was mentioned, "The cooperation with scientific research institutes is also based on clinical needs and research and development based on the advantages of Shenda endoscopy."
Looking back, the expansion of Shenda's endoscope product pipeline in the past is actually inseparable from the combination with clinical needs. "Initially starting from gynecology and urology, it is precisely because of the large number of patients with diseases that there is an urgent need. At that time, there was still a lack of relevant domestic enterprises to launch endoscope products that meet the needs." Today, Shenda's endoscope products have almost Covering all parts of the human body, when creating products for the department, "Shenda Endoscope (still) will choose to establish a relationship with the director of the department in advance, and then conduct market research, conduct research and development in terms of improving product functions and performance, and make product positioning. Choose." Chen Chong introduced.
Keep up with the consumables and domestic substitution, and strive to build a leading endoscope enterprise
The impact of the epidemic has made the hospital infection department pay more attention to the problem of nosocomial infection.
In fact, the industry has a long history of research on the possible cross-infection of endoscopes. Due to the complex structure, disinfection and cleaning cannot be completely completed, which often easily leads to cross-infection between patients, and cleaning, drying, and disinfection will greatly increase the operating cost of the hospital, all of which cause the repeated use of endoscopes. Limitations in clinical use.
Shen Da's endoscope undoubtedly saw the existence of this problem.
In 2017, Shenda endoscope obtained the registration certificate of three types of medical devices - "the world's first 'endoscope disposable protection system'". This system has successively won the "National High-tech Industrialization Demonstration Project" and "National Key New Product".
"Endoscope Disposable Protection System" of Shenda Endoscope
At present, the electronic duodenal endoscope being developed by Shenda Endoscope can be adapted to the single-use protection system. By ensuring that the electronic duodenal endoscope does not always come into contact with the patient's body cavity and body fluids, cross-infection caused by failure of disinfection can be effectively avoided.
At present, disposable endoscopes will undoubtedly be a major trend in the development of endoscopes.
In addition to the "world's first 'disposable endoscope protection system'" started in 2017, Shenda Endoscope is also carrying out the market layout of disposable endoscopes, completing technical reserves, and realizing productized research and development based on consumables. Its disposable cystopyeloscope is expected to obtain a Class III medical device registration certificate in 2023.
Under the current trend of domestic substitution of imported products for medical devices, Shenda Endoscopy is undoubtedly aware of the possible impact of this outlet.
"Import substitution of rigid endoscopes is a differentiated upgrade. What we want to provide to doctors is a complete set of solutions, not a single product." Chen Chong pointed out.
At present, the endoscope market in tertiary hospitals is still dominated by imported endoscopes. Jiang Shouwang also bluntly stated that it is also the goal that Shenda Endoscopy is constantly pursuing to recognize the advantages of imported product quality. "In the past two years, Shenda Endoscopy has also done a lot of work, including cooperating with domestic and foreign experts and famous research institutes. Some products have also entered the stage of clinical approval. To achieve domestic substitution, we must first ensure that the products reach foreign countries. First class."
This year, Shenda Endoscopy is also continuing to accelerate its cooperation with scientific research institutes, such as the gastroenterology photodynamic therapy cancer project in cooperation with Beijing 301 Hospital, which has also entered the clinical approval stage.
In response to the trend of digitalization, Shenda endoscopy is also making further efforts in "Shen Dayun" (collecting and further intelligent processing of patient detection images).
With the improvement of health awareness and the level of medical technology, people will pay more attention to the early detection of lesions in the future, and use minimally invasive methods for treatment. Its advantages are obvious - smaller incisions, shorter surgery time, fewer surgical side effects, and lower prices. Minimally invasive treatment requires endoscopes. Currently, 90% of the endoscope market is occupied by foreign companies. Rigid endoscopes are mainly monopolized by German and Japanese companies. The price is 6-10 times that of domestic products. import substitution space.
Jiang Shouwang mentioned that Shenda's endoscope, which has accumulated technical strength, will continue to respond to more intense market competition through product upgrades, and by "improving its own product line and improving its own product functions, the product quality will reach the international first-class product level." , and strive to be the industry leader and realize domestic substitution of imported products.”
Scan the QR code to read on your phone

E-mail : overseas@china-endoscope.com
Tel : 0086-24-88093290 Fax: 0086-24-88903118
NEWS
Services Support
CONTACT US
© 2022 Shenyang Shenda Endoscope Co., Ltd. All rights reserved. 辽ICP备05007908号-1 Powered by 300.cn


